
A University of Queensland team has shown that common laboratory animals are poor stand-ins for people when testing a major class of snake venom’s effect on blood.
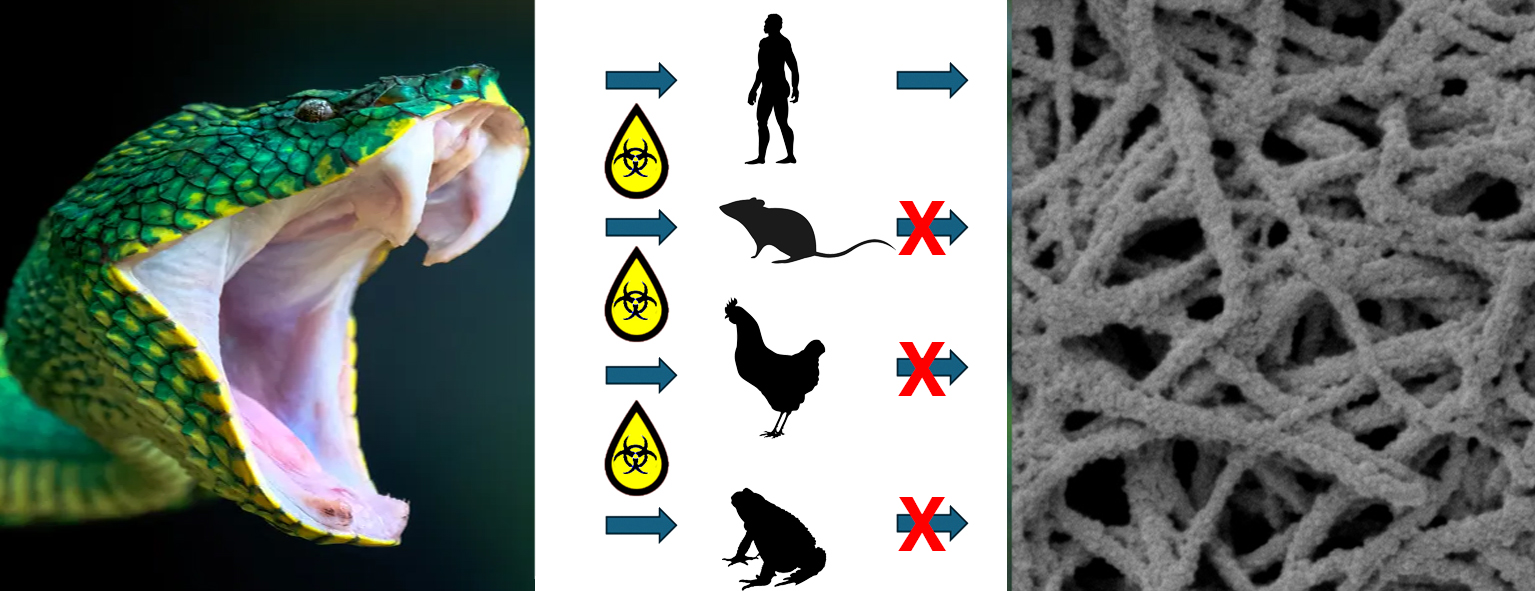
Researchers tested venoms from a wide range of medically important Asian pit viper snakes using a technique known as thromboelastography – a blood coagulation test – across human, rodent, avian, and amphibian blood plasmas.
Professor Bryan Fry, who leads UQ’s Adaptive Biotoxicology Lab, said the team were looking out for thrombin-like enzymes (TLEs), a type of protein studied for their potential as research tools, diagnostic agents, and therapeutics.
“Thrombin-like enzymes from snake venoms have long attracted interest as drug leads,” Professor Fry said.
“They’ve been explored to improve microcirculation, dissolve pathological clots, and reduce blood viscosity in conditions such as acute ischaemic stroke or deep vein thrombosis.
“Some venom-isolated enzymes have also reached clinical evaluation or limited clinical use, and they are widely used as diagnostic reagents in haematology.
“In this study, we found that several Asian pit viper venoms acted in a thrombin-like way on human plasma.
“But at the same doses in rodent, avian, and amphibian plasmas those same venoms did not behave in a thrombin-like manner.
“Instead, they behaved quite differently – producing a wide range of unrelated actions, from breaking down clotting material, to blocking clotting, to actually turning clotting on.”
Professor Fry highlighted that clinicians and researchers have leaned on animal models to anticipate human risk, but this study shows this is a shaky foundation for thrombin-like venoms.
“What looks procoagulant or what looks anticoagulant in an animal can act very differently in a patient,” he said.
“If we predict venom effects from animal models, we risk being misled – animal-only testing can misread both benefit and risk.
“A venom protease that appears to form strong clots in an animal model may actually produce weak, short-lived fibrin in humans, tipping patients toward bleeding through fibrinogen loss.
“Conversely, an enzyme that seems inert in rat may show potent, clinically relevant activity on human fibrinogen.
“Drug-development programmes that rely on animal plasma endpoints can therefore overestimate procoagulant usefulness, underestimate bleeding liability, or select the wrong candidates altogether.
“Our message for translational science is simple – if you want to know what will happen in people, test on human blood and report the right metrics - clot strength and lysis - not just clot onset.
“And we have many practical recommendations, such as anchor venom screening and antivenom assessment in human plasma with strength and lysis endpoints.
“Use animal plasmas to answer evolutionary questions, not as default predictors of human patient outcomes.
“And avoid over-interpreting animal data when designing adjunct therapies or inferring clinical directionality.”
The work has been published in the journal Toxicon.



